Insights
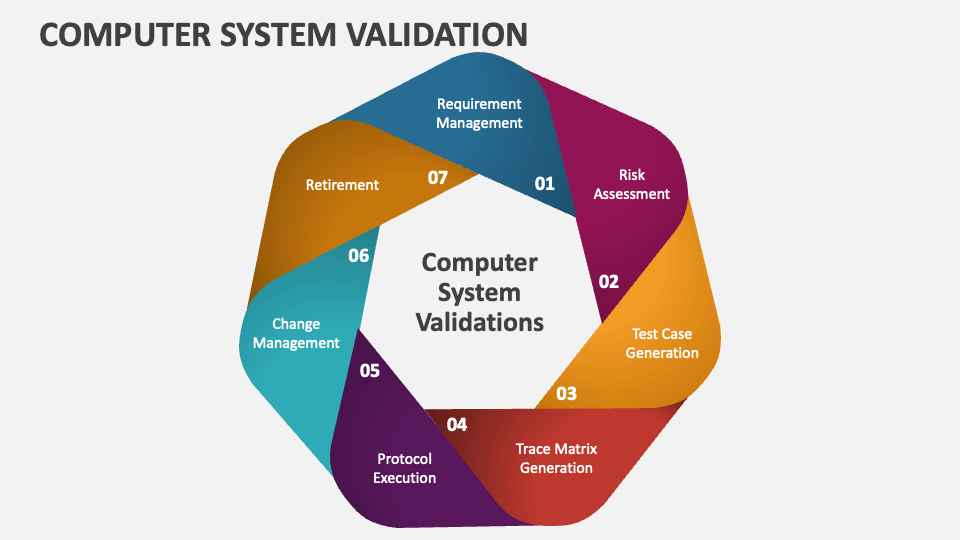
What is Computerized System Validation (CSV)?
In today’s life sciences industry, technology plays a crucial role in ensuring product quality, patient safety, and regulatory compliance. From laboratory instruments and manufacturing equipment to enterprise software and cloud-based solutions, computerized systems are everywhere.
But with great reliance on technology comes the responsibility to ensure that these systems are reliable, accurate, and compliant with global regulations. This is where Computerized System Validation (CSV) comes into the picture.
Definition of CSV
Computerized System Validation (CSV) is a documented process of ensuring that any software, hardware, or computerized system used in a regulated environment consistently produces results that meet predefined quality, compliance, and business requirements.
In simpler terms, CSV ensures your systems do what they are supposed to do, every single time, without compromising data integrity or compliance.
Why is CSV Important?
CSV is not just a regulatory requirement, but also a business necessity. Here’s why:
Regulatory Compliance
Many international regulatory agencies require that your systems are validated before they are put into use in regulated environment.
Data Integrity
Ensures that the data generated or handled by the validated systems complies with ALCOA+ (Attributable, Legible, Contemporaneous, Original, Accurate, Complete, Consistent, Enduring, Available) principles.
Audit Readiness
Proper CSV ensures companies are always prepared for inspections and audits.
Risk Management
Identifies and mitigates potential failures before they impact product quality or patient safety.
Operational Efficiency
A validated system runs smoothly, reducing downtime, errors, and rework.
How We Can Help
At Assure GxP Solutions, we provide end-to-end CSV services tailored for pharmaceutical companies. Whether it’s validating a new enterprise software, qualifying lab equipment, or remediating existing systems, our team ensures your systems are compliant, efficient, and audit-ready.
Explore our CSV Services to learn more about how we can support your compliance journey.

Drop Us a Line.


Let’s Build Future Together.

